Ask any kid, and most of them can tell you the three things that plants need to grow and thrive, each in appropriate measure: Sunlight. Water. Soil.
Most soils around here are either an ancient sand dune or an ancient swamp, with not much in between.
Supporter Spotlight
The sandier soils, like on the beach, tend to be limier.
The darker, muckier soils tend to be more acidic.
And neither of those tendencies is set in stone.
More important than your soil type is knowing the pH of that soil. What is pH, and why is the second letter capitalized and not the first?
A simple definition of pH is, it’s a measure of how acidic or base a substance is, as determined by the concentration of hydrogen ions. The higher the concentration of hydrogen ions, the more acidic your soil is. The fewer hydrogen ions there are means your soil is more alkaline. On a 14-point scale, battery acid is zero, with water or blood being neutral, and caustic substances like drain cleaner or soda being 14. Thus, 7 would be neutral, anything under 7 is acidic, and anything over 7 is alkaline.
Supporter Spotlight
As for why the second letter is capitalized, pH stands for power/potential of Hydrogen. H is capitalized because it stands for Hydrogen, and elements of the periodic table are generally capitalized, which makes for weird sentences.
A plant’s pH is what enables it to absorb nutrients.
Hydrangeas are a great natural litmus test for your soil. If you have hydrangeas, the bluer or more purple the blooms are, the more acidic your soil is. If your hydrangeas are pink, your soil is more alkaline.
If you want, you can put sulfur on one side of your hydrangea and lime on the other. Sulfur will lower the pH, while lime will raise it. The change won’t be instant, because altering your soil pH is not a quick thing, but you’ll end up with blue blooms on one side and pink on the other, shading to a delightful mix in the middle.
Our mostly acidic soil is why, even if you plant a pink hydrangea, it’s probably going to turn into a blue-flowered plant.
Why is pH important?
Some plants like acidic soil, some thrive in more neutral soil, some like more lime.
The easiest way I’ve found to explain pH is this: If you go to a restaurant and order a glass of sweet tea, if that restaurant fixes tea the right way … cooking the tea and mixing the sugar in while the tea is hot, everything is copacetic. If they give you a glass of ice tea and packets of sugar, no matter how much sugar you add or how much you stir, stir, stir … the tea will never absorb the sugar. The sugar will sit in the bottom of the glass, there but useless.
Plants are like that glass of tea. If the pH of your soil isn’t right for the type of plant you’re trying to grow, no matter how much fertilizer you throw at it, like that sugar sitting uselessly in the bottom of your glass of tea, the plant isn’t able to absorb the nutrients.
How do you know what your pH is, and how do you change it?
There are inexpensive kits similar to pool testers. All you need to use those is a bit of your soil, a bit of water, preferably bottled, and one of the capsules included in the test kit. Then you simply match the color of your concoction to the provided chart. Those are a good general indicator, and there are also more precise, probe-type, handheld pH meters, something like a meat thermometer.
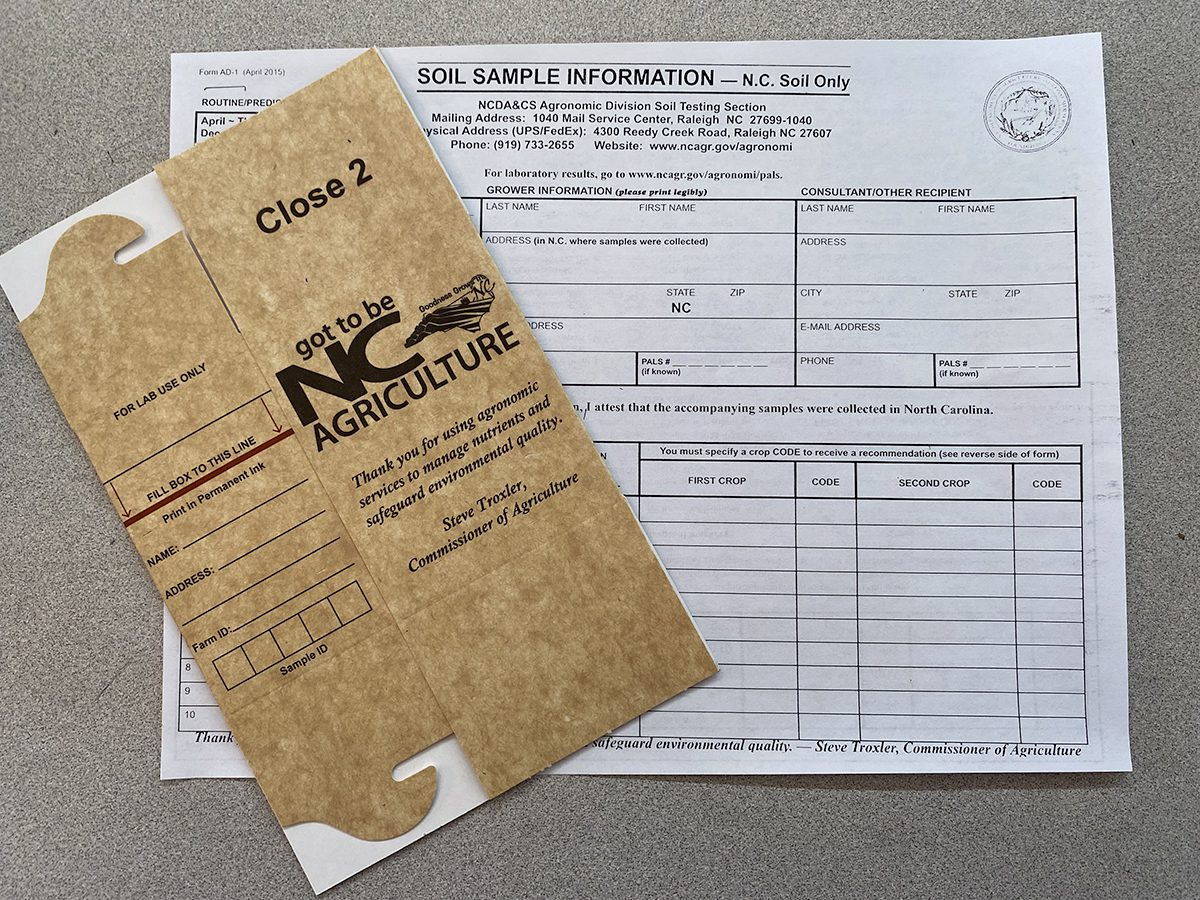
Better still, a more accurate way to test your soil is to pick up some soil sample boxes along with an information sheet and send both to the soil testing section of the North Carolina Department of Agriculture. As long as you send in your samples BEFORE Thanksgiving and AFTER April, there is no charge except postage. There is a charge in effect during the winter months because farmers are given priority then. In about three weeks, you’ll have your results!
We try to keep the sample boxes at the Newport Garden Center, or you can swing by your local Ag Extension office. The results will tell you exactly how much of which fertilizer is needed, whether you’re trying for a golf-course-worthy lawn or aiming for the prizewinning giant whatever in your garden.
Fall is the perfect time to send in your samples, because whether you need to add lime or sulfur, both need a while to take effect.
Putting out either without a soil test is like adding salt to something you have yet to taste. You might end up with an okay meal, or you might need to add more, or it might turn out to have so much salt it’s crunchy.
Not only can a pH imbalance inhibit plants from absorbing nutrients, it can also cause toxicity by allowing plants to absorb too much of the wrong thing.

A pH imbalance is why you can’t successfully plant things like blueberries and figs, for example, in the same area. Blueberries demand an acidic soil, and figs thrive on limey soil. Put the two together and it’s like trying to put a penguin and a monkey on an iceberg. One’s going to be extremely happy and the other … well, it isn’t going to live long.
Things that can affect pH are soil type, amount of rainfall, fertilizers, vegetation, temperature, and organic matter.
Most of the time you can correct pH by amending the soil. Sometimes the pH is just too far out of whack to correct it to the degree needed. In that case, it’s better to just give in and grow something else. For instance, centipede grass likes a low pH. If your pH is really high, you might be better off trying to grow something that likes a higher pH. Same with gardens.
Penguins or monkeys? You decide.