BEAUFORT – New research from Duke University suggests that there is more to the story of ocean acidification. Scientists have long believed that acidity in the ocean remains relatively stable under natural conditions, but researchers from the Duke Marine Laboratory in Beaufort documented dramatic natural short-term fluctuations in the water’s pH near Beaufort Inlet. These short-term fluctuations were so extreme that the researchers suggest they could play an important role in the effects of long-term ocean acidification on coastal organisms.
Supporter Spotlight
“What we saw over the annual or daily cycle was that those short-term natural sources of variability are just as extreme as long-term changes,” explained Zackary Johnson, Arthur P. Kaupe Assistant Professor in Molecular Biology at Duke’s Nicholas School of the Environment, and lead author on the research published in December in PLOS ONE.
 Zachary Johnson |
To understand why Johnson and his colleagues believe these short-term fluctuations could be something we should be concerned about you have to first understand what is happening to our ocean. In a nutshell: Increasing levels of carbon dioxide in our atmosphere are being absorbed by the ocean. These excess levels of carbon dioxide dissolved in the water cause chemical reactions to occur that both increase the acidity of the water and reduce saturation states of biologically important calcium carbonate minerals.
And if you think that this won’t have an impact on many organisms, think again. Calcium carbonate is the building block for the skeletal structure and shells of thousands of species of organisms. If you enjoy eating oysters or snorkeling over coral reefs then this is something you should be concerned about.
Supporter Spotlight
Some estimates predict that the surface of the ocean will be 150 times more acidic than it is today by the end of the century. But Johnson and his colleagues found evidence that there are pH changes just as dramatic already occurring over a much shorter time frame. Water samples taken over the course of a year from Piver’s Island, where the marine lab is located, showed variability in acidity that exceeds predictions of the expected change in global ocean acidity for the next 100-years.
“The idea is we know that the ocean is going to become more acidified. But there might also be variability in both the short-term and long-term, and for much of the ecosystem, the variability is just as important as the long-term (changes)”, said Johnson.
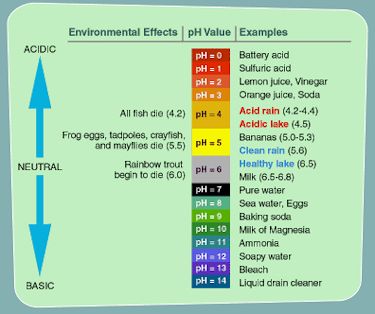 Source: EPA |
Johnson and his colleagues collected hourly to daily water samples and were surprised to find that there were huge fluctuations in the acidity of the water. These short-term fluctuations are influenced by a number of natural factors, including temperature, tidal flow and biological activity.
The effect of these short-term fluctuations, by themselves, is likely minimal.
“I am guessing that, at least those short-term changes, over an hour or a day, are largely caused by biology – plants and animals recycling carbon dioxide and oxygen over the course of a day. (This research) suggests that organisms are used to those changes. In the coastal environment at least, they may be adapted to short-term changes.” said Dana Hunt, assistant professor of microbial biology at the Nicholas School of the Environment and co-author on this research.
But what concerns these scientists is what the effects will be when these natural fluctuations are combined with a steady long-term increase in acidity.
As Hunt explains, the delicate balance that coastal organisms are adapted to may be thrown off by a more global shift in ocean conditions. “These changes could be additive,” she said. “They are fine under conditions now, but if baseline shifts occur they (organisms) will become more stressed or pushed past a threshold that they may already be at.”
Exactly what the effects will be of ocean acidification on organisms that are already coping with extreme ranges in water acidity is not clear.
Johnson notes that there is a possibility that adaptations to short-term acidic fluctuation could cause organisms to become more resilient in the face of ocean acidification. But he isn’t holding his breath for a widespread positive outcome.
“There are two ways this could go,” he said. “It could be good news. If organisms are already used to dealing with these changes then they might already be adapted to long-term acidification. The other possibility is not good news. If those short-term fluctuations already stress them out then how will they respond to an increasingly more acidic environment? We don’t know.”
 Dana Hunt |
As is true with most things in life, there will likely be winners and losers.
“There are probably going to be some winners who like it and some (species) who are losers. Who is going to win and who is going to lose – we just don’t know yet,” said Johnson.
Because phytoplankton takes in carbon dioxide they could benefit from ocean acidification by increasing their population and potentially creating large algal blooms.
Scientists are already aware of who some of the losers may be. Coral reefs rely on calcium carbonate to build their skeletal structure and by the end of the century increased acidity may cause reefs to dissolve faster than they are able to be built. Numerous commercial species such as oysters, clams and lobsters are unable to properly grow under more acidic conditions. And the effects of the very small, such as zooplankton, have far reaching effects up to the very large. Small pteropods, or “sea butterfly,” are a tiny sea creature about the size of a small pea whose shells dissolve in more acidic water. They are a source of food for everything from krill to salmon to some species of whales.
What Johnson and his colleagues are sure of is that coastal ecosystems are in for a change.
“We know it is going to get more acidic and this variability will be on top of that. But we don’t know exactly what the impact will be in our local ecosystem” said Johnson.
Johnson notes that this study was at only a single, coastal location and it is possible that other areas see less or more acidic variability. He hopes to extend his research to better understand exactly how organisms are already coping with these natural fluctuations, and to extend it to the open ocean where the variability is not so great but where ocean acidification is steadily increasing.
Ocean Acidification 101
Staff Report
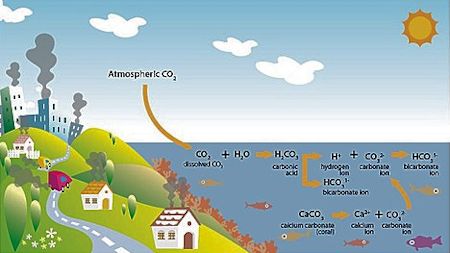
As it is absorbed into the atmosphere, C02 bonds with seawater, forming carbonic acid. This acid then releases a bicarbonate ion and a hydrogen ion. The hydrogen ion bonds with free carbonate ions in the water, forming another bicarbonate ion. The free carbonate would otherwise be available to marine animals to build calcium carbonate shells and skeletons. Graphic: Oceana |
When carbon dioxide, or CO2, is absorbed by seawater, chemical reactions occur that reduce the water’s pH, carbonate ion concentration and saturation states of biologically important calcium carbonate minerals. These chemical reactions are called “ocean acidification.”
Calcium carbonate minerals are the building blocks for the skeletons and shells of many marine organisms. In areas where most life now congregates in the ocean, the seawater is supersaturated with these minerals. Marine organisms, like oysters, clams, crabs and lobsters, that need these minerals to build their skeletons and shells have plenty of material to work.
Increasing ocean acidification is causing many parts of the ocean to become less saturated with these minerals, which is likely to affect the ability of some organisms to produce and maintain their shells.
Almost all atmospheric scientists think that the increased CO2 is due to the burning of coal, oil and other fossil fuels. Since the beginning of the Industrial Revolution in the late 1800s, the pH of surface ocean waters has fallen by 0.1 pH units. Since the pH scale, like the Richter scale, is logarithmic, this change represents about a 30 percent increase in acidity.
Current predictions indicate that the oceans will continue to absorb carbon dioxide and become even more acidic. If little is done to decrease fossil-fuel emissions, the surface waters of the oceans by the end of this century could be nearly 150 percent more acidic, resulting in a pH that the oceans haven’t experienced for more than 20 million years.







